Solution/Service:
A system that offers the flexibility to fill pill bottles with various types of medications, in both tablet and capsule forms, while eliminating the risk of cross-contamination and ensuring complete traceability throughout the process.
Project Challenge:
The objective was to design a system that minimizes the time required to introduce new medicines to the market.
Project Description
Scotland’s new Medicines Manufacturing Innovation Centre has received the Just In Time (JIT) Clinical Trials Manufacturing System, a groundbreaking technology aimed at expediting the market introduction of new medicines. Upon completion of the installation, this system will enable the MMIC to fill pill bottles with different drug types, in tablet or capsule form, without the risk of cross-contamination, while ensuring full traceability throughout the process.
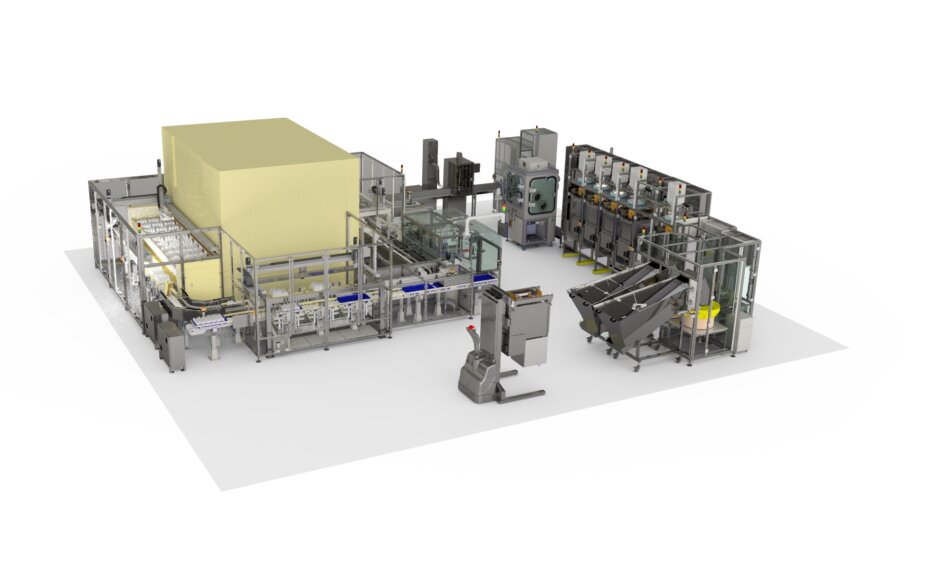
This multi-million-pound turnkey system consists of several individual modules, each designated for specific operations such as handling, filling, sealing, weighing, marking, labelling, and packing. A distinctive feature of this system is its capability to adhere to stringent pharmaceutical hygiene and cleanliness standards without requiring the entire system to be situated in an ISO-class clean room environment.
The system build project necessitated not only the specification and integration of a diverse range of proprietary technologies but also creative design solutions to address various technical challenges across the system’s components.
System Capability
The system is designed to handle and process two sizes of pill containers supplied in bulk and fed into the system from bulk hoppers. Each hopper can accommodate either 1,000 or 2,000 bottles, depending on the size, with the bottles passing through bowl feeding, orientation, and singulation systems for individual loading onto the line
Product traceability
Product traceability is a critical aspect of any medical or pharmaceutical setting. Each bottle is laser-marked with a unique 2D matrix code for tracking and verification throughout the automated processes. The initial filling operation involves loading a desiccant capsule into each bottle within a humidity and temperature-monitored filling module. To confirm the correct amount of desiccant is added, precise tare and fill weights are recorded and validated against the product matrix code identifier to ensure compliance with the expected specifications for each bottle.
Subsequent humidity and temperature-monitored drug filling modules, each functioning as independent units designed for quick changeover between pharmaceutical product types, dispense tablets or capsules of varying drug types and strengths. Tablets or capsules are fed through the system in a controlled manner, with accurate counts recorded in a holding chamber. Before the drugs are introduced into the pill bottle, the bottle ID marker is re-checked to ensure the correct association between the container and the product. The processes within these modules—related to locating bottles, feeding drugs, monitoring the environment, and preventing cross-contamination—eliminate the necessity for the entire system to be housed in an ISO-class controlled clean room. This aspect of the system is currently under patent applications.
Once the pill containers contain both desiccant and tablets or capsules, the 2D ID code is read again before they are check-weighed. If the weight is correct, they proceed to the next module, where a foil seal is applied to the top of the bottle using induction heating.
Bottle and Tray Storage and Retrieval
Following this, each bottle has a child-proof cap fitted and tightened to a predetermined torque at the next module. In addition to the operations described for handling, filling, sealing, and capping bottles, the system includes extensive bottle and tray storage, retrieval, and labelling capabilities. This allows for the handling, storage, and processing of finished products in various ways, including transferring completed products from the bottle infeed system to trays, moving bottles from a white stock infeed to trays, and retrieving bottles from previously populated and stored trays for transfer to the labelling module. Bottles can also be picked and loaded into shipping trays, which are transported to the dispatch area once filled to the appropriate level. The storage component of the system can accommodate over 17,000 bottles
Bottle labelling
Bottle labelling occurs in a separate, self-contained system capable of accepting both bottle sizes and applying either a single panel label or a wrap-around label as needed. The 2D code on each bottle is scanned before its unique label is printed, verified by machine vision, and subsequently applied.
This comprehensive and adaptable system ensures traceability throughout the manufacturing process, incorporating secure reject areas at each stage to guarantee that only verified products advance to the next operation. The system is also designed for scalability to meet future capacity needs and to facilitate the introduction of alternative packaging formats and component variants.

